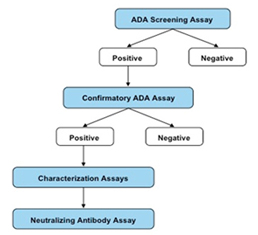
Immunogenicity assays
- Development and Validation of Method according to EMEA and FDA guidelines.
- Analysis of Samples in GLP controlledenvironment.
- Development and validation of Method for Neutralizing Antibodies.
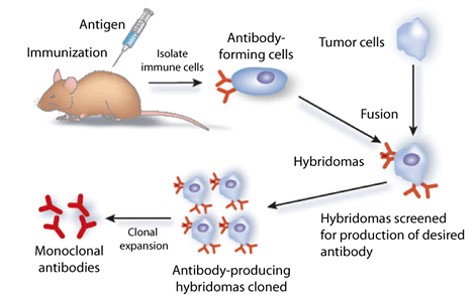
Generation of Monoclonal Antibodies(Mab’s)
- Development of Monoclonal and polyclonal Antibodies.
- We Take development of high Quality antibodies against therapeutic Proteins, Peptides and Small Molecules.
- Development and validation of Method for Neutralizing Antibodies.
Pharmacokinetic Assays
We have industry leading experience in development and validation of PK/TK assay for large molecules and peptides.
- Develop and validate assays that have analytical ranges which appropriate for the study samples.
- Develop assay that are both specific and sensitive.
- Provide support for critical reagent generation and purification including the generation of antibodies to be used for capture and detection reagents.
- Assess method feasibility in multiple technology platforms.
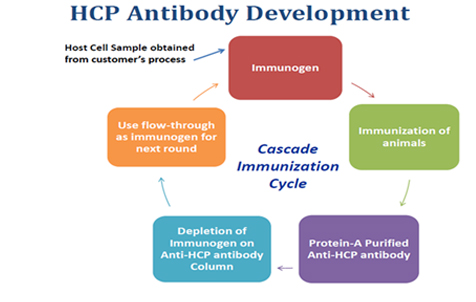
Development and Validation of Host Cell Protein Assay
Host-cell proteins (HCPs) constitute a major part of process-related impurities during biologics production. The amount of residual HCPs in drug product is generally considered a critical quality attribute (CQA), due to their potential to affect product safety and efficacy. Therefore, it is a regulatory requirement to monitor the removal of HCPs in drug product during bioprocess development.